Kenya administered its first jabs against Covid-19 in March 2021, following the delivery of a million doses of the Oxford-AstraZeneca vaccine.
Weeks earlier, local media had reported on the vaccine’s approval for use in the country.
As vaccinations started, health minister Mutahi Kagwe said the Oxford vaccine’s “efficacy and safety had been approved by the World Health Organization (WHO)”.
“On our part, the ministry of health recommends approval via stringent regulatory authority and registration by the pharmacy and poisons board for any vaccine to come into our country,” Kagwe said on 10 March.
Since then Russia’s Sputnik 5 vaccine has also been given the official nod, while Kagwe said the pharmacy board is evaluating the Pfizer-BioNtech and Sinovac vaccines.
But how are vaccine approvals done in Kenya? Our factsheet answers this and other questions.
Kenya’s pharmacy board regulates drugs and drug trials
The pharmacy and poisons board is mandated by Kenyan law to “regulate the practice of pharmacy and the manufacture and trade in drugs and poisons”.
The country’s national policy on immunisation says that “all vaccines for human use in Kenya” must meet quality requirements as determined by the board, which also approves them for public use.
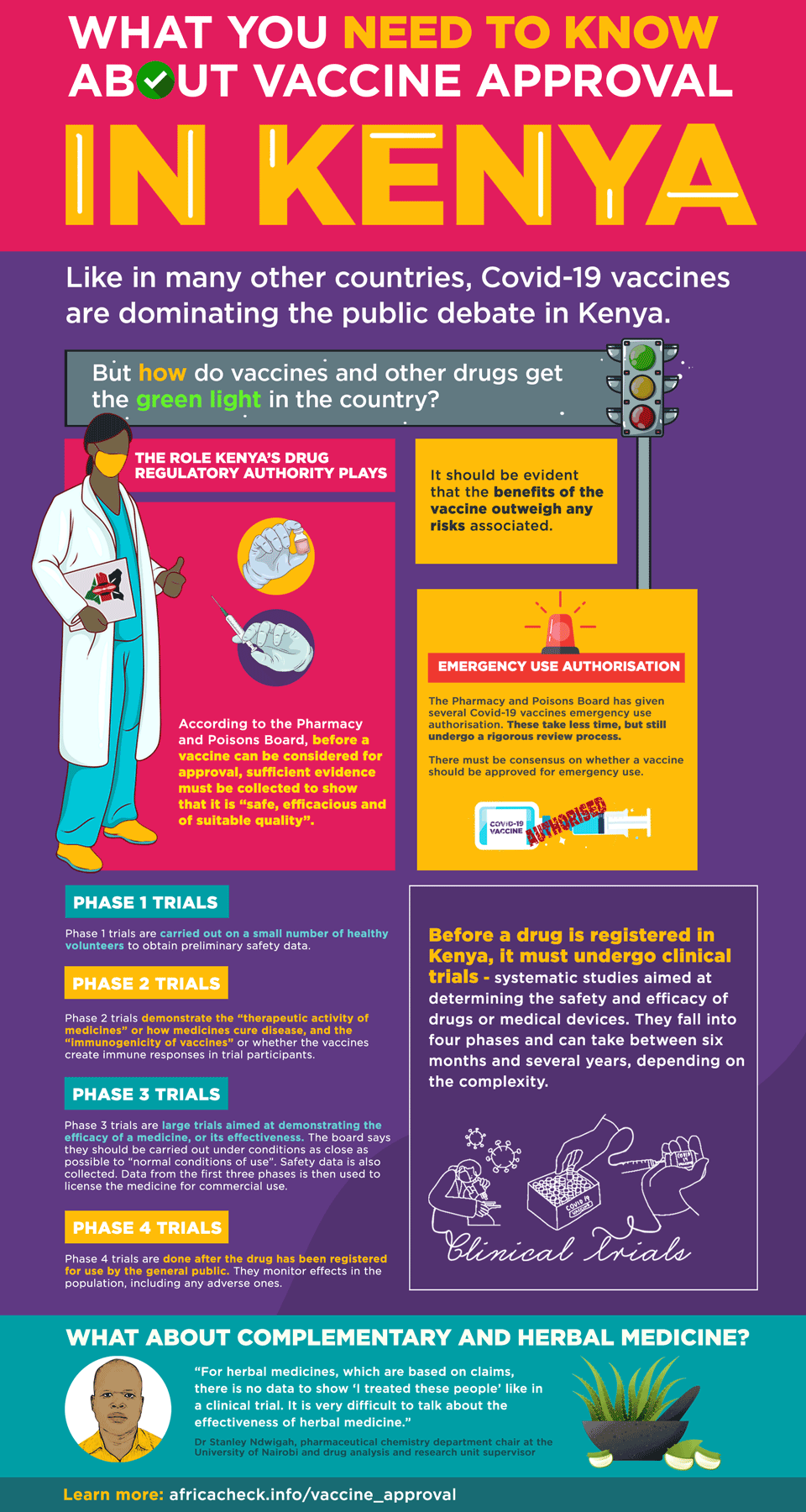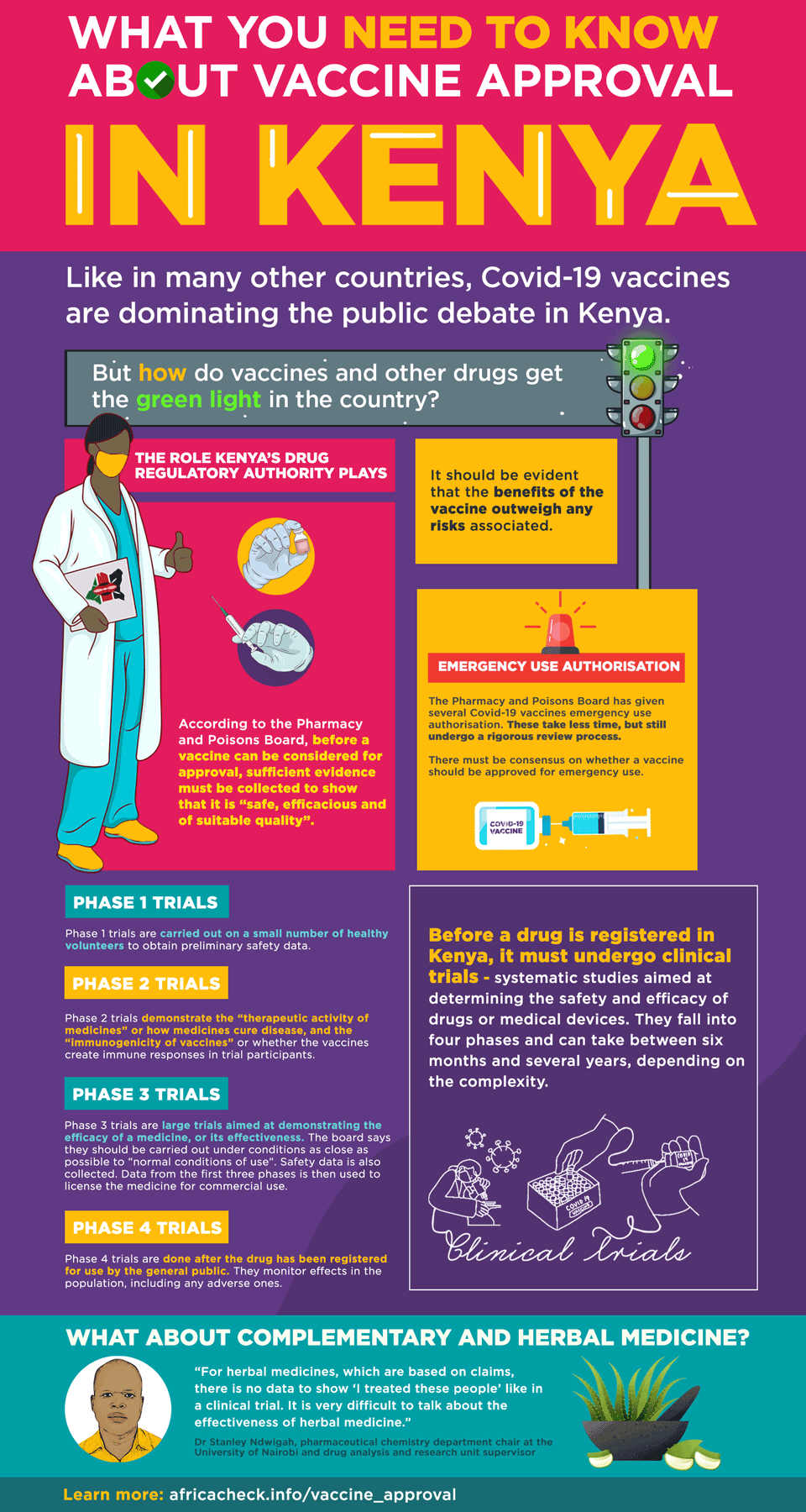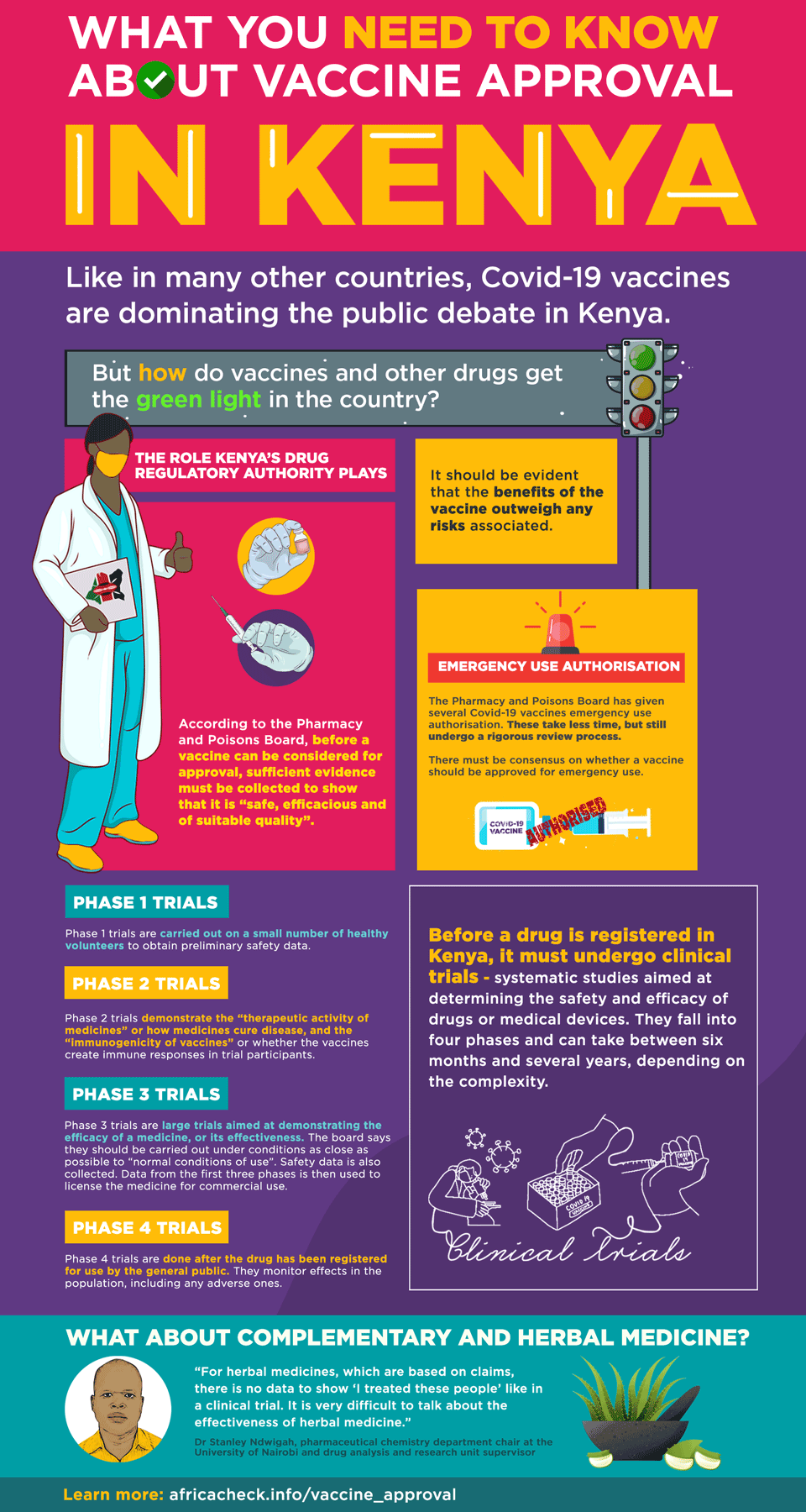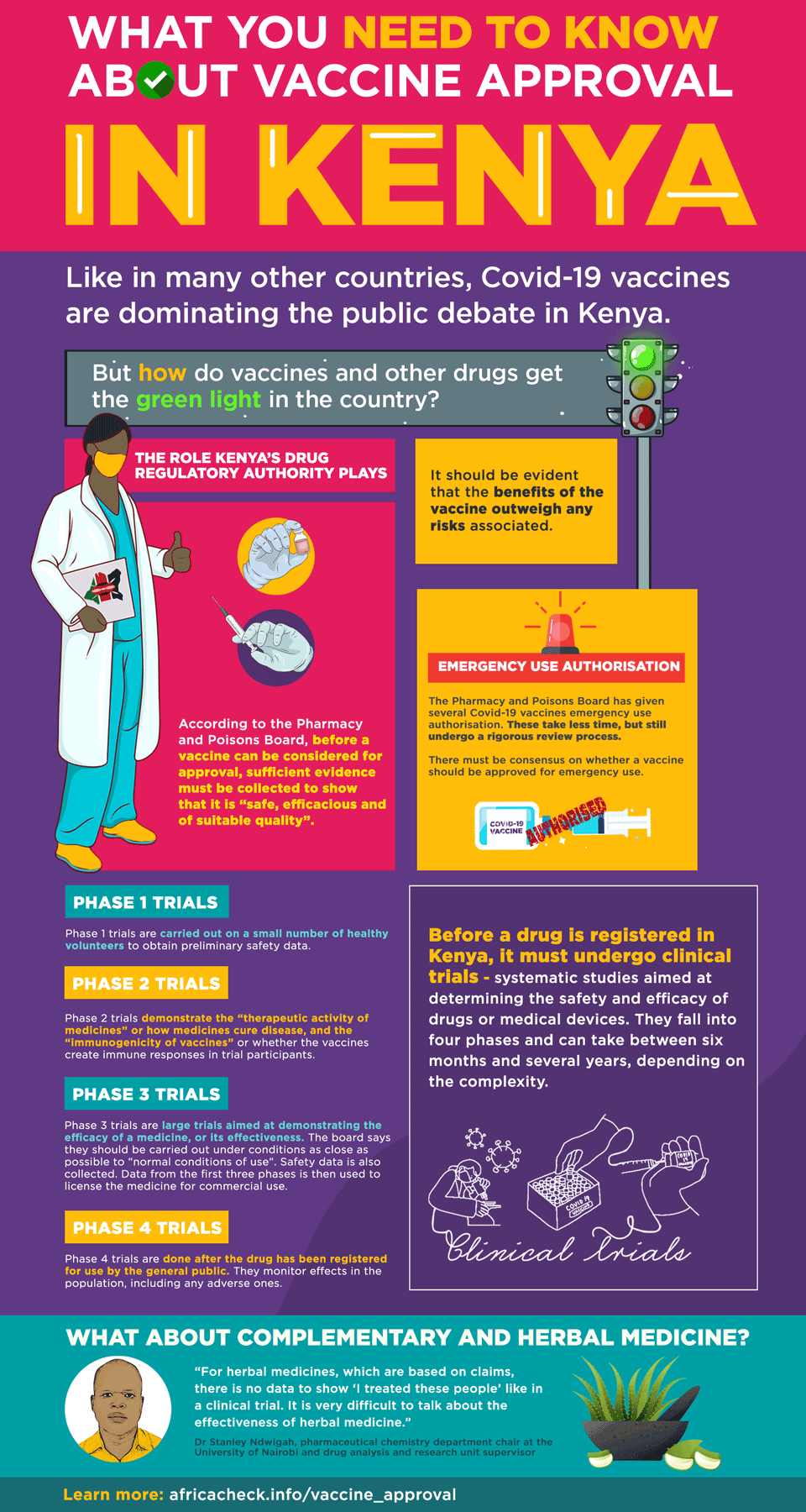
According to 2018 guidelines from the agency, “before a vaccine is considered for approval, sufficient scientific and clinical evidence must be collected to show that it is safe, efficacious and of suitable quality”.
This evidence “includes results from human clinical trials and for acceptance; it should be evident that the benefits of the vaccine outweigh any risks associated”.
Following the Covid-19 pandemic, the pharmacy board issued rules on clinical trials in the country, including by Oxford-AstraZeneca.
Board collaborates with researchers
Ensuring the safety of medicines in the country is “a collaborative effort”, Dr Peterson Muriithi, lecturer in community health at the University of Nairobi’s school of public health, told Africa Check.
In addition to the pharmacy board, the drug analysis and research unit and the Kenya Medical Research Institute, or Kemri, are involved.
The drug analysis unit is a research laboratory in the University of Nairobi’s department of pharmaceutical chemistry that carries out “routine analysis on pharmaceutical products as well as market surveillance”.
Kemri is the national body responsible for carrying out health research.
Four phases of clinical trials
Dr Peter Ikamati is the acting chief principal regulatory officer at the pharmacy and poisons board. He also oversees the regulatory and safety monitoring of Kenya’s vaccination effort.
Before a drug is registered in Kenya, it must undergo clinical trials, he told Africa Check.
The board defines clinical trials as “systematic studies aimed at determining the safety and efficacy of drugs or medical devices”. They fall into four phases:
-
Phase 1 trials are carried out on a small number of healthy volunteers to obtain preliminary safety data.
-
Phase 2 trials demonstrate the “therapeutic activity of medicines” or how medicines cure disease, and the “immunogenicity of vaccines” or whether the vaccines create immune responses in trial participants. These are also “routinely done in patients”.
-
Phase 3 trials are large trials aimed at demonstrating the efficacy of a medicine, or its effectiveness. The board says they should be carried out under conditions as close as possible to “normal conditions of use”. Safety data is also collected. Data from the first three phases is then used to license the medicine for commercial use.
-
Phase 4 trials are done after the drug has been registered for use by the general public. They monitor effects in the population, including any adverse ones.
Trials usually multi-centre, though all phases possible in Kenya
Trials can “take years or as short a period as six months. It depends on the complexity”, Ikamati of the pharmacy board said. Some researchers also apply to do the second and third phases in Kenya, after having done the first phase in other countries.
This did not mean Kenya could not do phase 1 trials. “Our law allows us to approve any phase of clinical trials,” he said.
Trials could also be prolonged, Ikamati said, giving the example of the anti-malaria “RTS,S” vaccine which has taken many years and is now in phase 4. This happened because the formulation and dosage had changed so as to make it more effective and reduce adverse effects.
“Usually, clinical trials will be multi-centre, which means they are conducted within several countries, because you want to see the efficacy and safety effects in various populations,” Ikamati said.
Though all medicines must be registered, not all require clinical trials to be performed in Kenya, he said.
“Innovator products or new chemical products will require clinical trials. Due to our economic situation, most of these will be advanced in developed countries, anyway. However, those owners or sponsors can also apply to conduct clinical trials in Kenya.”
Emergency use
In announcing Covid-19 vaccine approvals, the board said it had given them “emergency use authorisation” and that these are not registrations.
There are two paths to reach such a decision, Ikamati told Africa Check.
-
A “reliance mechanism” that relies on reports from stringent regulatory authorities such as the US Food and Drug Administration, the UK’s Medicines and Healthcare Products Regulatory Agency, Health Canada and Australia’s Therapeutic Goods Administration.
-
A “ full dossier” method where all information on the product is submitted in the common technical document format, including quality and clinical data that reflects efficacy and safety.
For the recently granted emergency approval for the AstraZeneca vaccine in Kenya, the board “used a full dossier assessment”, Ikamati told Africa Check. “We were in constant communication with the WHO in Geneva on the steps taken.”
WHO gave emergency use approval for this vaccine 15 February.
The Sputnik 5 vaccine was also subjected to “a full assessment of the common technical dossier document”, Ikamati said.
“It is not mandatory to have local analysis of products in emergency periods and also for WHO-prequalified or emergency use-listed products,” Ikamati said.
Bharat Biotech of India has applied for approval of its “Covaxin” vaccine, while the board has held pre-submission meetings with other manufacturers, he said.
The board also looks at pharmacovigilance requirements which are a requirement for emergency use medicine. “Companies have to give a risk management plan on how to report adverse reactions,” he said.
For the Oxford-AstraZeneca vaccine, Ikamati said the board is receiving reports on adverse effects through its pharmacovigilance system. “For Kenya we’ve not had any events of blood clotting, or thromboembolic events, as reported in Europe,” he said.
To approve or not
Dr Stanley Ndwigah chairs the pharmaceutical chemistry department at the University of Nairobi and supervises the drug analysis and research unit.
He told Africa Check that emergency authorisations take a shorter time, sometimes only a month. “The documents are looked at, by a committee and not a single person. Then there must be a consensus on whether to approve or not.”
If there are questions the applicants have to address them. The data provided will show the efficacy, safety and quality, he said. And in the clinical trials there must be analysis that “shows reasonable evidence that the medicine works”.
The drug analysis unit analysed some samples to ensure there are no “bacterial contaminants or toxins”, Ndwigah said, in addition to examining their quality.
Complementary medicines also vetted
Herbal or complementary medicine practitioners are registered by the ministry of culture and not health, Ikamati said.
Currently, the pharmacy and poisons board receives applications for herbal and complementary medicines. These products are taken through a similar vetting process as for conventional medicines or chemicals, he said.
The experts said that while the quality and safety of complementary products could be monitored, it was much more challenging to measure efficacy.
“For herbal medicines, which are based on claims, there is no data to show ‘I treated these people’ like in a clinical trial. It is very difficult to talk about the effectiveness of herbal medicine,” Ndwigah of the drug analysis and research unit said.
“For herbal medicines we are interested in the safety and the quality, not efficacy.” This included ensuring they did not have fungal or bacterial contaminants and that dangerous residues were absent.
Another challenge was dosage, Ikamati of Kenya’s pharmacy and poisons board said. “Some trees and herbs will have differences in concentration depending on the location or the season.
“You might find the molecule which is able to treat is in high concentration during the hot seasons. In the cold season it has a lower concentration. Unless you are measuring it in the laboratory, you can’t really be sure whether you are giving someone an overdose or underdose.”
“There needs to be a professional authority regulating herbal practitioners, which we currently do not have in Kenya,” Ikamati said.



Add new comment